Engineering Autonomy for Human Health
The Urgent Need for Organ Innovation
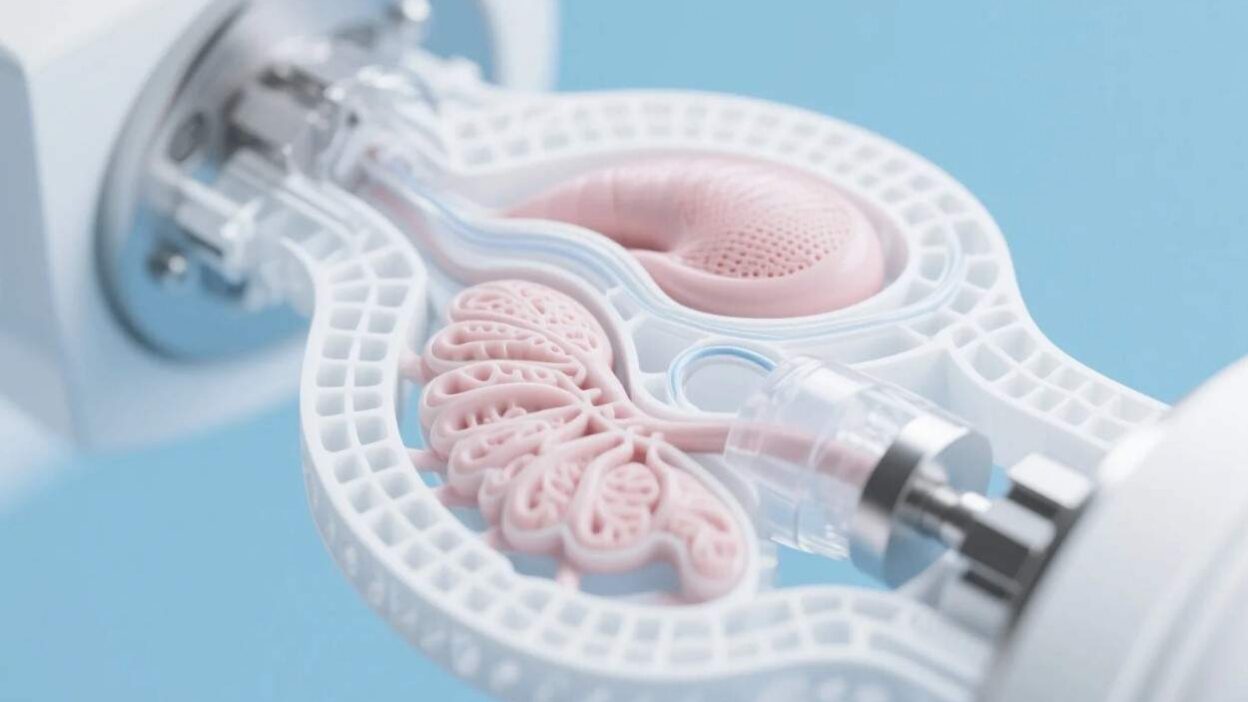
Organ failure is a global crisis. Over 17 million people die annually from end-stage organ disease, with millions more on transplant waiting lists—often for years. Traditional organ transplants rely on scarce donor organs, face immune rejection risks, and require lifelong immunosuppressive drugs. Enter closed-loop artificial organs: self-sustaining, functional organs engineered to operate independently, without external support. Powered by 3D bioprinting—a technology that constructs living tissues layer by layer using biological materials—these organs promise to revolutionize transplantation, offering personalized, rejection-free solutions. This article explores how 3D bioprinting is enabling closed-loop artificial organs, their potential to transform healthcare, and the challenges and future of this groundbreaking field.
Closed-Loop Artificial Organs: Defining Autonomy in Medicine
A closed-loop artificial organ is a bioengineered structure that mimics the form and function of a natural organ while maintaining self-regulation. Unlike traditional prosthetics or static tissue grafts, these organs:
- Self-Sustain: They generate their own energy (e.g., via embedded capillaries for nutrient delivery) and repair minor damage.
- Function Independently: They perform physiological tasks (e.g., pumping blood, filtering toxins) without external pumps or machines.
- Adapt to the Body: Engineered with a patient’s own cells, they minimize immune rejection.
The “closed-loop” moniker refers to their ability to operate as a self-contained system, mirroring the body’s natural homeostasis. For example, a 3D-printed heart would not only beat but also regulate its rhythm, respond to hormonal signals, and repair damaged tissue—just like a healthy human heart.
3D Bioprinting: The Tool That Builds Life
3D bioprinting is the cornerstone of closed-loop organ engineering. It merges additive manufacturing (3D printing) with cell biology, using specialized “bioinks” to construct living tissues. Here’s how the process unfolds:
1. Design: From Imaging to Digital Blueprints
First, medical imaging (MRI, CT scans) or 3D modeling software creates a digital blueprint of the target organ, capturing its precise structure (e.g., the branching airways of a lung or the valves of a heart).
2. Bioink Preparation: The Building Blocks of Life
Bioinks are cell-laden materials that mimic the extracellular matrix (ECM)—the scaffold that supports cells in the body. Common bioinks include:
- Hydrogels: Water-rich polymers (e.g., alginate, collagen) that provide a nurturing environment for cells.
- Decellularized Extracellular Matrix (dECM): Derived from donor tissues, dECM retains growth factors and structural proteins, enhancing cell compatibility.
- Synthetic Polymers: Biodegradable materials (e.g., polycaprolactone) that degrade over time, leaving only natural tissue.
3. Printing: Layer by Layer, Cell by Cell
Using robotic printers, bioinks are deposited in precise layers, guided by the digital blueprint. Multi-material printers can integrate different cell types (e.g., cardiac muscle cells, endothelial cells for blood vessels) and structural components (e.g., rigid scaffolds for bone).
4. Maturation: Bioreactors and Vascularization
Freshly printed tissues are immature and lack functional blood vessels. Bioreactors—incubators that mimic the body’s environment (temperature, oxygen, nutrient flow)—stimulate cell growth and vascularization. Advanced bioreactors use dynamic mechanical forces (e.g., pulsatile flow) to train cells to behave like natural tissues, a critical step for larger organs like the heart or liver.
Achieving Closed-Loop Functionality: The Key to Autonomy
For an organ to operate independently, it must replicate three core functions:
1. Vascularization: The Lifeline of Life
Blood vessels are the organ’s “highways,” delivering oxygen and nutrients. 3D bioprinting now integrates vascular networks into printed tissues. For example:
- Microfluidic Channels: Engineers embed tiny, branched channels in bioinks, which mature into functional blood vessels when seeded with endothelial cells (the cells lining blood vessels).
- Co-Printing with Endothelial Cells: By embedding endothelial progenitor cells alongside structural cells, researchers create self-assembling vascular networks that connect to the host’s circulatory system.
2. Self-Regulation: Mimicking the Body’s Feedback Loops
Closed-loop organs must respond to internal and external signals. For instance:
- Cardiac Regulation: A 3D-printed heart, engineered with pacemaker-like cells, could adjust its beating rate in response to the body’s demand for oxygen.
- Liver Metabolism: A bioengineered liver might sense toxin levels and upregulate detoxification enzymes, mimicking the liver’s natural adaptive responses.
3. Immunocompatibility: Avoiding Rejection
Using a patient’s own cells (autologous cells) or stem cells reprogrammed to match their immune profile minimizes rejection. For example, researchers at Wake Forest Institute for Regenerative Medicine have printed skin grafts using a patient’s own skin cells, which integrate seamlessly without immune attacks.
Current Progress: From Labs to Clinical Trials
While full-sized, fully functional closed-loop organs remain experimental, 3D bioprinting has achieved remarkable milestones:
- 3D-Printed Hearts: In 2023, researchers at Carnegie Mellon University printed a small, functional heart model using human heart cells. The organ beat rhythmically in a bioreactor, with integrated microchannels for nutrient delivery.
- Bioengineered Lungs: Harvard University’s Wyss Institute developed a 3D-printed lung model with alveoli (air sacs) and blood vessels, capable of oxygenating blood in animal trials.
- Cartilage and Bone: Clinics worldwide now use 3D-printed cartilage grafts for joint repairs, with some designs integrating stem cells to promote long-term integration.
These advancements highlight the technology’s potential to bridge the gap between lab research and clinical application.
Challenges: The Road to Widespread Adoption
Despite progress, significant hurdles remain:
1. Vascularization at Scale
Larger organs (e.g., kidneys, livers) require complex, branched vascular networks. Current bioprinting techniques struggle to replicate this complexity, often leading to poor nutrient delivery and cell death in the organ’s core.
2. Long-Term Functionality
Most printed organs degrade over time, as the body’s immune system gradually breaks down the scaffold or cells lose their function. Researchers are exploring “smart” bioinks that degrade slowly or stimulate continuous cell regeneration.
3. Regulatory and Ethical Hurdles
Regulatory bodies (e.g., FDA) require extensive safety and efficacy testing for 3D-printed organs. Questions about long-term outcomes, off-target effects, and the ethics of “designer organs” (e.g., enhancing organ function beyond natural limits) also slow adoption.
4. Cost and Accessibility
3D bioprinting is expensive, with costs driven by specialized equipment, bioinks, and labor. Ensuring equitable access—particularly for low-income regions—is a critical challenge.
The Future: Organs as Self-Sustaining Ecosystems
The future of closed-loop artificial organs lies in innovation and collaboration:
- Advanced Bioinks: Synthetic bioinks with built-in growth factors or drug-eluting capabilities could enhance tissue regeneration and function.
- AI-Driven Design: Machine learning models will optimize organ architecture, predicting how modifications (e.g., vessel density, cell density) affect performance.
- Organ-on-a-Chip: Miniaturized, closed-loop organ models (e.g., a “heart-on-a-chip”) will accelerate drug testing and personalized medicine, reducing reliance on animal trials.
- Global Partnerships: Initiatives like the 3D Bioprinting Alliance are fostering collaboration between academia, industry, and regulators to standardize protocols and accelerate approval.
Redefining Life Through Engineering
Closed-loop artificial organs, enabled by 3D bioprinting, represent a paradigm shift in healthcare. By engineering self-sustaining, patient-specific organs, we are moving closer to a world where organ failure is no longer a death sentence. While challenges like vascularization and regulation persist, the progress made in labs and clinics today offers hope for millions.
As Dr. Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine, once said, “3D bioprinting is not just about building organs—it’s about building hope.” With continued innovation, closed-loop artificial organs could redefine medicine, making organ transplantation accessible, safe, and personalized for all.
Join the conversation at AIDNESS. Should 3D-printed organs be prioritized in healthcare policy? How can we ensure these technologies are accessible to all? Share your thoughts—we’re all building a future where no one waits for a second chance at life.