Unlocking the Microbiome’s Role in Longevity
The Aging Crisis and the Forgotten Organ
Aging is the universal human experience, marked by a gradual decline in physical and cognitive function. While modern medicine has extended lifespan, it has done little to slow the biological aging process itself—a phenomenon driven by cellular senescence, chronic inflammation, mitochondrial dysfunction, and a weakened immune system. Enter the gut microbiome: the trillions of microorganisms residing in the digestive tract, once dismissed as mere “gut flora,” now recognized as a critical regulator of health and aging.
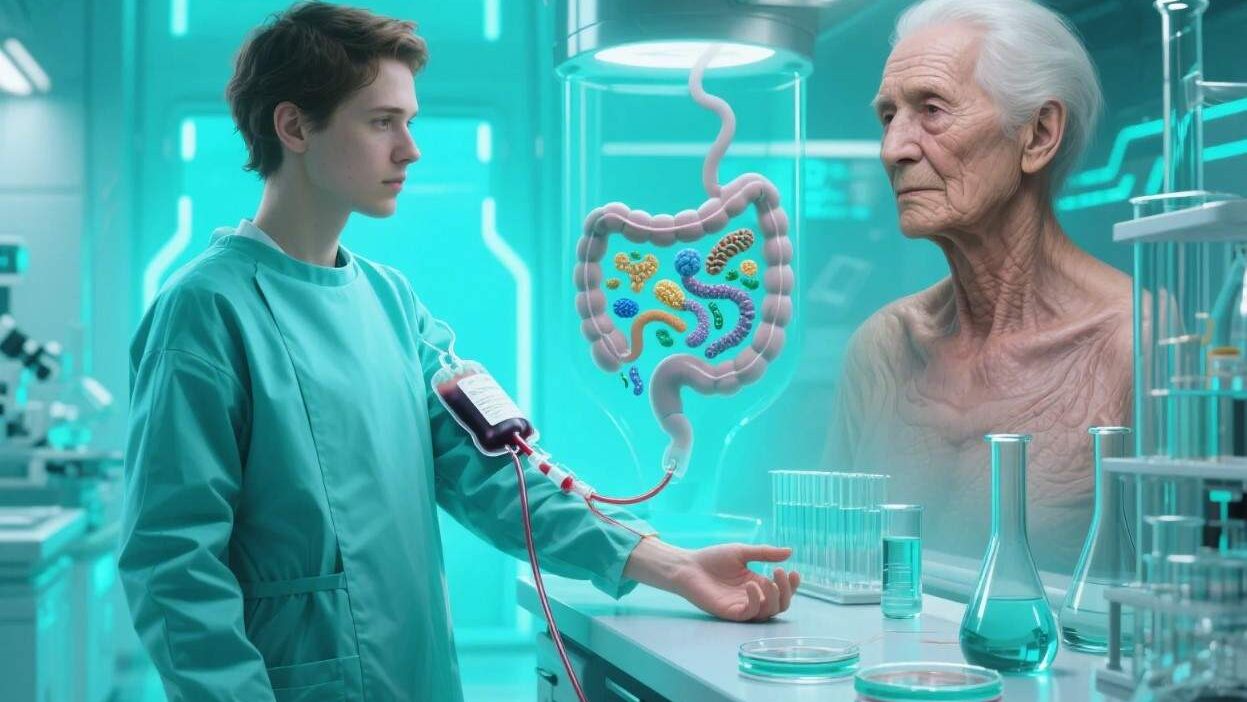
Recent breakthroughs suggest that transplanting a “young” gut microbiome into older individuals—via fecal microbiota transplantation (FMT)—could reverse age-related decline. This radical approach, borrowed from treating infections like Clostridioides difficile (C. diff), is now being explored as a potential fountain of youth. This article delves into the science, promise, and challenges of using gut microbiome transplants (GMT) to combat aging.
The Gut Microbiome: A Master Regulator of Aging
The gut microbiome is far more than a collection of bacteria; it’s a dynamic ecosystem that interacts with nearly every organ system. Key functions include:
- Digestion: Breaking down complex carbohydrates and producing nutrients like short-chain fatty acids (SCFAs).
- Immunity: Training immune cells to distinguish friend from foe, reducing chronic inflammation.
- Metabolism: Regulating energy balance, insulin sensitivity, and lipid profiles.
- Brain-Gut Axis: Producing neurotransmitters (e.g., serotonin) and metabolites that influence mood and cognition.
Aging disrupts this delicate balance. Studies show that as we age:
- Microbiome Diversity Declines: Beneficial bacteria (e.g., Akkermansia muciniphila, Faecalibacterium prausnitzii) diminish, while pro-inflammatory species (e.g., E. coli, Clostridium) thrive.
- Gut Barrier Dysfunction: The intestinal lining weakens, allowing toxins and pathogens to leak into the bloodstream (“leaky gut”), triggering systemic inflammation.
- Metabolic Shifts: Reduced SCFA production impairs energy regulation, while elevated bile acids and lipopolysaccharides (LPS) drive insulin resistance and oxidative stress.
These changes accelerate aging by promoting cellular damage, impairing organ function, and fostering age-related diseases like Alzheimer’s, diabetes, and cardiovascular disease.
Gut Microbiome Transplants (GMT): How They Work
GMT involves transferring fecal matter from a healthy, younger donor into the gastrointestinal tract of an older recipient. This procedure, refined over decades for C. diff treatment, aims to:
- Restore Microbiome Diversity: Reintroduce beneficial bacteria that decline with age.
- Repair Gut Barrier Function: Strengthen the intestinal lining, reducing “leaky gut” and systemic inflammation.
- Modulate Metabolism: Boost SCFA production and regulate bile acid levels, improving energy use and reducing oxidative stress.
Mechanisms of Action
- Beneficial Bacteria: Donor microbiota include species like A. muciniphila, which strengthens the gut barrier by producing mucin (a protective gel), and F. prausnitzii, which generates anti-inflammatory SCFAs like butyrate.
- Microbial Metabolites: Short-chain fatty acids (SCFAs) reduce inflammation, while indoles (from tryptophan metabolism) activate sirtuins—enzymes linked to longevity.
- Immune Regulation: A balanced microbiome suppresses pro-inflammatory immune cells (e.g., T helper 17 cells) and enhances anti-inflammatory Tregs (regulatory T cells).
Current Research: Promises and Early Wins
While human trials are limited, preclinical and small-scale human studies suggest GMT could reverse aging biomarkers:
Animal Studies: Lifespan Extension
- Mice: A 2020 study in Nature Aging found that transplanting fecal matter from young mice into aged mice extended their lifespan by 15–20% and improved cognitive function. The treated mice showed reduced inflammation, healthier gut barriers, and increased SCFA levels.
- Worms: In C. elegans, GMT from young donors delayed age-related paralysis and extended lifespan by up to 30%, attributed to enhanced mitochondrial function.
Human Trials: Early Signs of Hope
- UC Davis Study (2022): Researchers transplanted fecal matter from healthy young donors (ages 18–30) into 15 older adults (ages 60–85) with age-related frailty. After 12 weeks, recipients showed:
- Reduced systemic inflammation (lower IL-6, TNF-α levels).
- Improved gut barrier function (reduced LPS in blood).
- Enhanced muscle strength and cognitive performance.
- OpenBiome’s “Aging Trial” (Ongoing): This initiative is testing GMT in older adults with mild cognitive impairment (MCI). Early data suggests recipients have slower cognitive decline and better metabolic health.
Challenges: Hurdles to Widespread Adoption
Despite promising results, GMT faces significant challenges:
1. Safety Concerns
- Pathogen Transmission: FMT carries risks of transferring harmful bacteria (e.g., multidrug-resistant E. coli) or viruses (e.g., SARS-CoV-2). Stringent donor screening (including stool pathogen testing) is critical.
- Unintended Effects: In rare cases, FMT has caused infections, autoimmune flare-ups, or metabolic imbalances. Long-term safety data in aging populations is lacking.
2. Efficacy Variability
Not all recipients respond equally. Factors like baseline microbiome health, diet, and genetics influence outcomes. For example, older adults with severe dysbiosis may require multiple transplants or adjuvant therapies.
3. Ethical and Regulatory Gaps
- Donor Consent: Who qualifies as a “healthy” donor? How are minors or marginalized groups represented in donor pools?
- Regulation: The FDA classifies FMT as an “investigational new drug,” requiring rigorous trials for each indication. Expanding access to GMT will demand updated guidelines.
4. Cost and Accessibility
FMT is expensive (up to $10,000 per procedure) and requires specialized facilities. Scaling production (e.g., via synthetic microbiomes or lyophilized capsules) could reduce costs, but this remains experimental.
The Future: GMT as a Staple of Anti-Aging Care
The future of GMT in aging lies in innovation and collaboration:
- Synthetic Microbiomes: Engineered probiotics or “microbiome pills” could deliver specific beneficial strains without the risks of FMT. Companies like Vedanta Biosciences are developing rationally designed microbial consortia.
- Personalized GMT: Using AI to analyze a recipient’s microbiome and tailor donor selection for optimal results.
- Combination Therapies: Pairing GMT with other anti-aging interventions (e.g., caloric restriction mimetics, senolytics) to amplify benefits.
Rewiring Aging Through the Gut
Gut microbiome transplants represent a paradigm shift in aging research, offering a tangible way to reverse age-related decline by restoring microbial balance. While challenges like safety and scalability persist, early studies suggest GMT could become a cornerstone of longevity medicine.
As we age, our bodies are not just “wearing out”—they are out of sync with the microbial partners that once kept us vital. By harnessing the gut microbiome, we may finally unlock the secret to aging gracefully.
Join the conversation at AIDNESS. Should GMT be prioritized in anti-aging research? How can we balance innovation with safety and equity? Share your thoughts—we’re all part of rewriting the story of aging.