Bridging the Gap Between Disability and Independence
The Silent Struggle of Paraplegia and the Promise of Neural Implants
Paraplegia, the partial or complete loss of motor and sensory function in the lower body, affects over 2.5 million people globally, often due to spinal cord injuries (SCI), strokes, or degenerative conditions like multiple sclerosis. For these individuals, even simple tasks—standing, walking, or maintaining balance—become insurmountable challenges. Traditional treatments, such as physical therapy or medications, offer limited relief, leaving many trapped in a cycle of dependency.
But a revolutionary solution is emerging: neural implants—surgically placed devices that bridge the gap between the brain, spinal cord, and limbs, restoring lost neural connections. By directly interfacing with the nervous system, these implants are redefining what it means to live with paraplegia, offering hope for restored mobility and independence. This article explores how neural implants work, their current impact, and the future of this transformative technology.
What Are Neural Implants? Types and Mechanisms
Neural implants are precision-engineered devices designed to interact with the body’s neural networks. For paraplegia, they target the spinal cord, peripheral nerves, or brain to bypass damaged pathways and restore function. Key types include:
1. Epidural Spinal Cord Stimulation (ESCS)
ESCS involves implanting a small device (a “pulse generator”) under the skin, connected to electrodes placed on the spinal cord’s outer membrane (dura). These electrodes deliver low-level electrical currents to stimulate spinal neurons, “waking up” dormant circuits that control leg movement.
- How It Works: By mimicking natural neural signals, ESCS activates the spinal cord’s ability to generate rhythmic leg movements (locomotion) without requiring input from the brain. Studies show it can enable stepping motions in patients with incomplete SCI.
2. Intraspinal Microstimulation (ISM)
ISM uses ultra-thin electrodes inserted directly into the spinal cord’s gray matter (where motor and sensory neurons reside). This allows for highly targeted stimulation of specific neural circuits.
- Advantage: Unlike ESCS, ISM can activate individual neurons, enabling finer control over leg muscles. Researchers at the Swiss Federal Institute of Technology (EPFL) used ISM to restore walking in rats with complete SCI, a breakthrough that inspired human trials.
3. Peripheral Nerve Interfaces (PNIs)
PNIs target the nerves that innervate the legs (e.g., the femoral or sciatic nerves). Electrodes are implanted near these nerves to directly stimulate muscle fibers, bypassing the spinal cord entirely.
- Application: PNIs are particularly useful for patients with intact peripheral nerves but damaged spinal cords. For example, a 2022 trial in Nature Medicine used PNIs to restore ankle movement in a paraplegic patient, allowing them to push off the ground while walking with a walker.
4. Brain-Computer Interfaces (BCIs) for Mobility
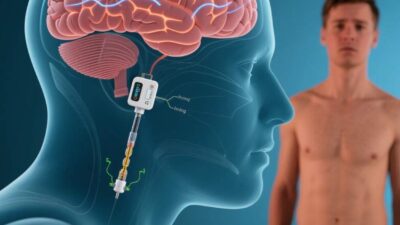
BCIs bridge the brain and spinal cord by decoding neural signals from the brain’s motor cortex (which controls movement) and translating them into commands for spinal stimulators or prosthetic limbs.
- Innovation: BCIs enable “intentional” movement—patients think about walking, and the device translates that thought into leg motion. In 2023, a team at the University of Pittsburgh demonstrated a BCI that allowed a paralyzed man to walk 10 meters using only his thoughts.
How Neural Implants Restore Mobility: The Science Behind the Solution
Neural implants address paraplegia at its root: disrupted neural communication. Here’s how they restore function:
1. Reactivating Dormant Spinal Circuits
After SCI, the spinal cord’s “central pattern generator” (CPG)—a network of neurons that coordinates walking—often remains intact but inactive. ESCS and ISM stimulate the CPG, reactivating its ability to generate rhythmic leg movements. A 2021 study in Cell Reports found that ESCS restored CPG activity in 70% of trial participants, enabling stepping motions.
2. Restoring Sensory Feedback
Paraplegia often involves losing touch and proprioception (awareness of limb position). Neural implants can deliver sensory feedback by stimulating sensory neurons, allowing patients to “feel” their legs. For example, PNIs that stimulate the dorsal root ganglia (sensory nerve clusters) enable patients to sense pressure or temperature, improving balance and coordination.
3. Enhancing Autonomic Function
Beyond mobility, neural implants can restore autonomic functions like bladder and bowel control, which are often disrupted in paraplegia. By stimulating nerves that regulate these systems, implants reduce reliance on catheters or medication, improving quality of life.
Clinical Applications: Real-World Success Stories
While neural implants are still in development, several trials and case studies highlight their potential:
- Case 1: The “Walking Man”
In 2023, a 35-year-old man with complete paraplegia (due to a motorcycle accident) became the first patient to walk unassisted using a BCI. The device, implanted in his skull and lower back, decoded signals from his motor cortex and sent commands to a spinal stimulator, enabling him to take 100+ steps in a lab setting. - Case 2: Restoring Independence
A 52-year-old woman with incomplete SCI (from a fall) participated in an ESCS trial. After 6 months of stimulation, she regained the ability to stand for 30 minutes and walk short distances with a cane, drastically reducing her reliance on a wheelchair. - FDA Approvals
In 2022, the FDA approved the Nevro Senza SCS system for chronic pain, but its off-label use for SCI mobility is growing. Similarly, the Medtronic Intellis SCS device is being tested in trials for restoring leg movement.
Challenges and Limitations
Despite progress, neural implants face significant hurdles:
1. Technical Hurdles
- Durability: Implantable devices must withstand years of use without degradation. Electrodes can corrode or shift, reducing efficacy.
- Signal Stability: Neural signals are noisy and variable; AI algorithms are needed to filter and interpret them accurately.
2. Biological Challenges
- Tissue Compatibility: The body often rejects foreign materials, leading to scar tissue (fibrosis) that disrupts neural connections. Coatings like hydrogels or drug-eluting films aim to reduce this risk.
- Scar Tissue Formation: Post-surgery scarring can insulate electrodes, limiting their ability to stimulate nerves.
3. Ethical and Accessibility Concerns
- Cost: Neural implants can cost 200,000, making them inaccessible to low-income patients. Insurance coverage varies widely.
- Patient Selection: Not all paraplegics are candidates—implants work best for those with intact spinal cord segments or peripheral nerves.
4. Psychological Barriers
Patients may fear surgery or struggle with the “uncanny” sensation of controlling limbs via a device. Long-term psychological support is critical.
The Future: Innovations and Hope
The future of neural implants for paraplegia lies in innovation and collaboration:
- Advanced Materials: Flexible, biodegradable electrodes (e.g., graphene-based) could reduce scarring and improve durability.
- AI-Driven Optimization: Machine learning will refine electrode placement and stimulation patterns, enhancing efficacy.
- Combination Therapies: Implants paired with stem cell therapy or exoskeletons could restore full mobility. For example, a 2024 trial combines ESCS with mesenchymal stem cells to regenerate spinal cord tissue.
- Democratization: Efforts by nonprofits (e.g., the Christopher & Dana Reeve Foundation) aim to reduce costs and expand access globally.
Restoring Mobility, Reclaiming Lives
Neural implants are more than a technological marvel—they are a lifeline for paraplegics, offering the promise of standing, walking, and living independently. While challenges like cost and durability persist, ongoing research and clinical trials are bringing this future closer.
As neuroscientist Dr. Grégoire Courtine, a pioneer in spinal cord stimulation, once said, “We’re not just fixing a broken spine—we’re redefining what it means to be mobile.” For the millions living with paraplegia, neural implants are not just devices—they are a bridge to a life unshackled by disability.
Join the conversation at AIDNESS. Should neural implants be prioritized in spinal cord injury research? How can we ensure these technologies are accessible to all? Share your thoughts—we’re all part of building a world where mobility is a right, not a privilege.