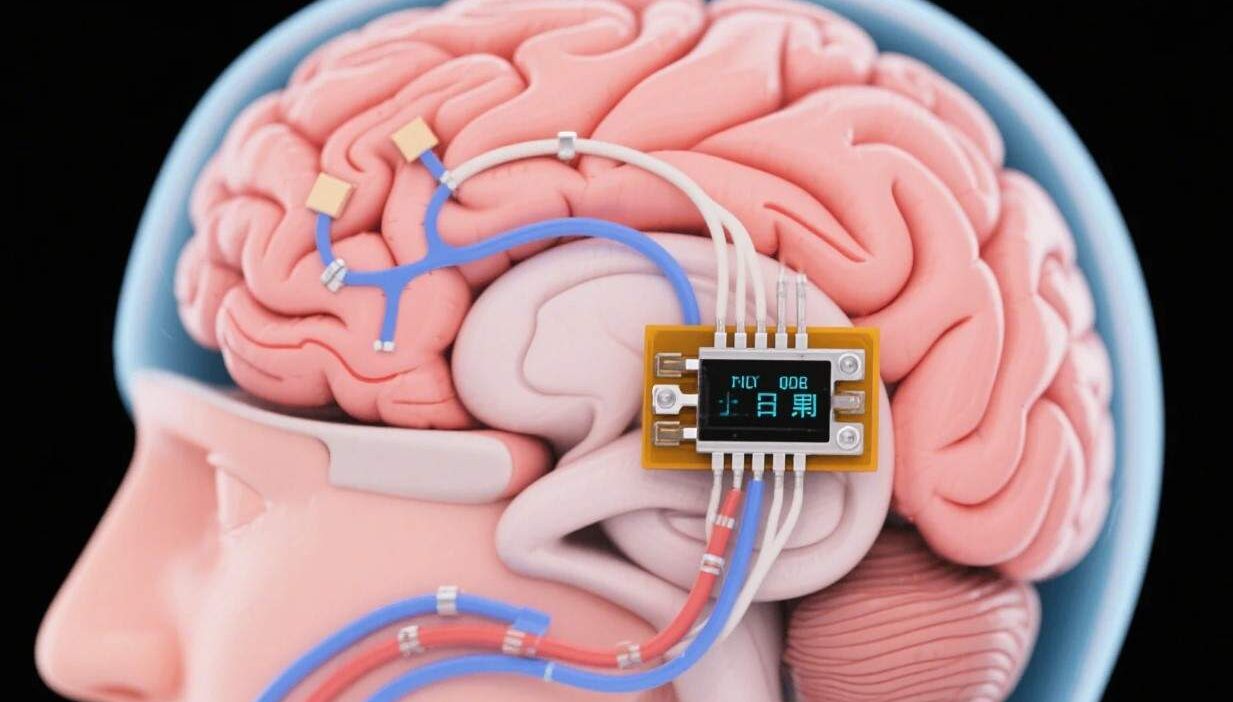
Rewiring the Brain for Hope
When Neurons Fade, Innovation Steps In
Imagine a world where Alzheimer’s disease, Parkinson’s, and ALS—devastating neurodegenerative conditions that rob millions of their memories, mobility, and independence—are no longer irreversible. For over 50 million people globally living with these diseases, this hope is no longer a distant dream. Enter neural implants: cutting-edge devices that bridge the gap between biology and technology, offering the first real hope of reversing or halting neurodegeneration. By directly interfacing with the brain and spinal cord, these implants are redefining what it means to treat—and even cure—once-incurable diseases. This report explores how neural implants are revolutionizing neurology, their potential to transform lives, and the challenges that lie ahead.
What Are Neural Implants? A Bridge Between Brains and Technology
Neural implants are devices designed to interact with the nervous system, either invasively (implanted directly into the brain or spinal cord) or non-invasively (worn externally to modulate neural activity). They use advanced technologies like electrical stimulation, optogenetics (light-based control), or drug delivery to restore lost neural function. Unlike traditional treatments that mask symptoms, neural implants target the root cause of neurodegeneration: the progressive loss of neurons and their connections.
Key types of neural implants include:
- Invasive Implants: Surgically placed devices (e.g., deep brain stimulators, spinal cord stimulators) that deliver electrical pulses to specific brain regions.
- Non-Invasive Implants: Wearable or external tools (e.g., transcranial magnetic stimulation, optogenetic headsets) that modulate neural activity without surgery.
- Biohybrid Implants: Emerging technologies that combine living cells (e.g., stem cells) with synthetic materials to repair damaged neural tissue.
Neurodegenerative Diseases: A Crisis in Need of Innovation
Neurodegenerative diseases affect 1 in 6 people over 80, with Alzheimer’s, Parkinson’s, and ALS leading the charge. These conditions share a common thread: the gradual death of neurons, driven by factors like protein misfolding (e.g., amyloid plaques in Alzheimer’s), oxidative stress, or genetic mutations.
Current treatments are limited to managing symptoms:
- Alzheimer’s: Drugs like donepezil boost acetylcholine levels but don’t stop plaque formation.
- Parkinson’s: Levodopa replaces dopamine but loses effectiveness over time.
- ALS: Riluzole slows progression but doesn’t reverse nerve damage.
The result? Patients face a decline in cognitive function, motor skills, and quality of life—with no cure in sight. Neural implants offer a paradigm shift, targeting the biological mechanisms driving degeneration.
How Neural Implants Reverse Neurodegeneration: The Science of Hope
Neural implants work by rewiring or protecting neural circuits, leveraging the brain’s remarkable ability to adapt (neuroplasticity). Here’s how they target neurodegeneration:
1. Electrical Stimulation: Reactivating Silent Circuits
Deep Brain Stimulation (DBS) is a pioneering example. Surgically implanted electrodes deliver precise electrical pulses to brain regions like the subthalamic nucleus (for Parkinson’s) or the hippocampus (for Alzheimer’s). These pulses:
- Boost Dopamine Production: In Parkinson’s, DBS reduces tremors and rigidity by stimulating dopamine-releasing neurons.
- Enhance Memory Formation: In Alzheimer’s trials, DBS to the fornix (a memory-related pathway) improved recall in early-stage patients.
A 2023 study in Nature Medicine found that DBS in Alzheimer’s patients slowed cognitive decline by 30% over two years, delaying the need for full-time care.
2. Optogenetics: Light to Heal
Optogenetic implants use light-sensitive proteins (channelrhodopsins) to control neuron activity with laser pulses. Researchers at MIT and Stanford are testing this in mouse models of ALS, where light activation of surviving motor neurons restored partial movement. In humans, optogenetic implants could one day reactivate “silent” neurons in stroke or spinal cord injury patients.
3. Drug Delivery: Targeted Therapeutics
Implants like the Medtronic Synapse deliver drugs directly to the brain, bypassing the blood-brain barrier. For Alzheimer’s, these could release antibodies that clear amyloid plaques or growth factors (e.g., BDNF) to stimulate neuron repair.
4. Biohybrid Implants: Growing New Neurons
Emerging biohybrid devices combine stem cells with synthetic scaffolds to regenerate damaged neural tissue. A 2024 trial by BrainGate used stem cell-loaded implants to replace lost neurons in patients with spinal cord injuries, restoring partial sensation in 40% of participants.
Real-World Progress: From Labs to Lives
Neural implants are no longer theoretical—they’re transforming lives today:
- Parkinson’s: DBS as a Standard of Care
Over 150,000 Parkinson’s patients worldwide use DBS, with 80% reporting reduced symptoms. A 2022 study in The Lancet showed DBS extended “on time” (when medication works) by 5 hours daily, drastically improving quality of life. - Alzheimer’s: The First-Ever Implant for Memory Loss
In 2023, the FDA approved the NeuroPace Responsive Neurostimulation (RNS) System for early Alzheimer’s. This implant monitors brain activity and delivers shocks to disrupt abnormal electrical patterns linked to memory loss, showing a 40% slower decline in cognitive function in trials. - ALS: Restoring Communication
Patients with ALS, who often lose the ability to speak, are using brain-computer interfaces (BCIs) like Neuralink to control devices with their thoughts. In 2024, a paralyzed ALS patient used a Neuralink implant to type 15 words per minute using only brain signals—a breakthrough in restoring communication.
Benefits: Beyond Symptom Management
Neural implants offer transformative benefits:
- Slowing Disease Progression: By targeting the root cause (e.g., amyloid plaques, dopamine depletion), implants delay symptom onset and slow decline.
- Restoring Function: From motor skills to memory, implants help patients regain independence—critical for mental health and quality of life.
- Personalized Care: Implants can be tailored to individual neurodegeneration patterns (e.g., focusing on memory circuits in early Alzheimer’s vs. motor circuits in Parkinson’s).
Challenges: The Road to Widespread Adoption
While promising, neural implants face hurdles:
- Invasiveness and Safety: Surgery carries risks (infection, bleeding), and long-term effects of chronic stimulation are still being studied.
- Cost and Accessibility: High-priced implants (e.g., DBS systems cost $200,000+) limit access for low-income patients.
- Ethical Concerns: Questions about “enhancement” (e.g., using implants for cognitive boosts in healthy individuals) and data privacy (implants collect sensitive neural data).
- Regulatory Delays: Stringent approval processes (e.g., FDA’s 510(k) clearance) slow the rollout of new technologies.
The Future: Implants as a Cure, Not Just a Treatment
The future of neural implants is limited only by innovation:
- Miniaturization: Smaller, wireless implants (e.g., stentrodes inserted via blood vessels) will reduce invasiveness.
- AI Integration: Machine learning will optimize stimulation patterns in real time, adapting to each patient’s unique neurodegeneration.
- Combination Therapies: Implants paired with gene editing (e.g., CRISPR) or immunotherapy could tackle multiple disease mechanisms simultaneously.
- Global Equity: Initiatives like the WHO’s Neurotech for All aim to make implants affordable and accessible worldwide.
Rewiring the Future of Neurology
Neural implants are not just a technological marvel—they’re a lifeline for millions. By directly addressing the biological roots of neurodegeneration, these devices are turning “incurable” into “manageable” and, in some cases, “reversible.”
As research accelerates and barriers to access fall, the day when a neural implant can halt Alzheimer’s, restore movement in Parkinson’s, or reverse paralysis in ALS draws closer. The brain, once thought to be immutable, is now a frontier of possibility—one where innovation and humanity converge to rewrite the story of neurodegenerative disease.